Building Research Initiative Group: chronic illness management and adHerence in Transplantation (BRIGHT)
The BRIGHT study is the first multi-center, multi-continental study examining healthcare system and heart transplant centers chronic illness management practice patterns and potential correlates of immunosuppressive medication non-adherence.
Background
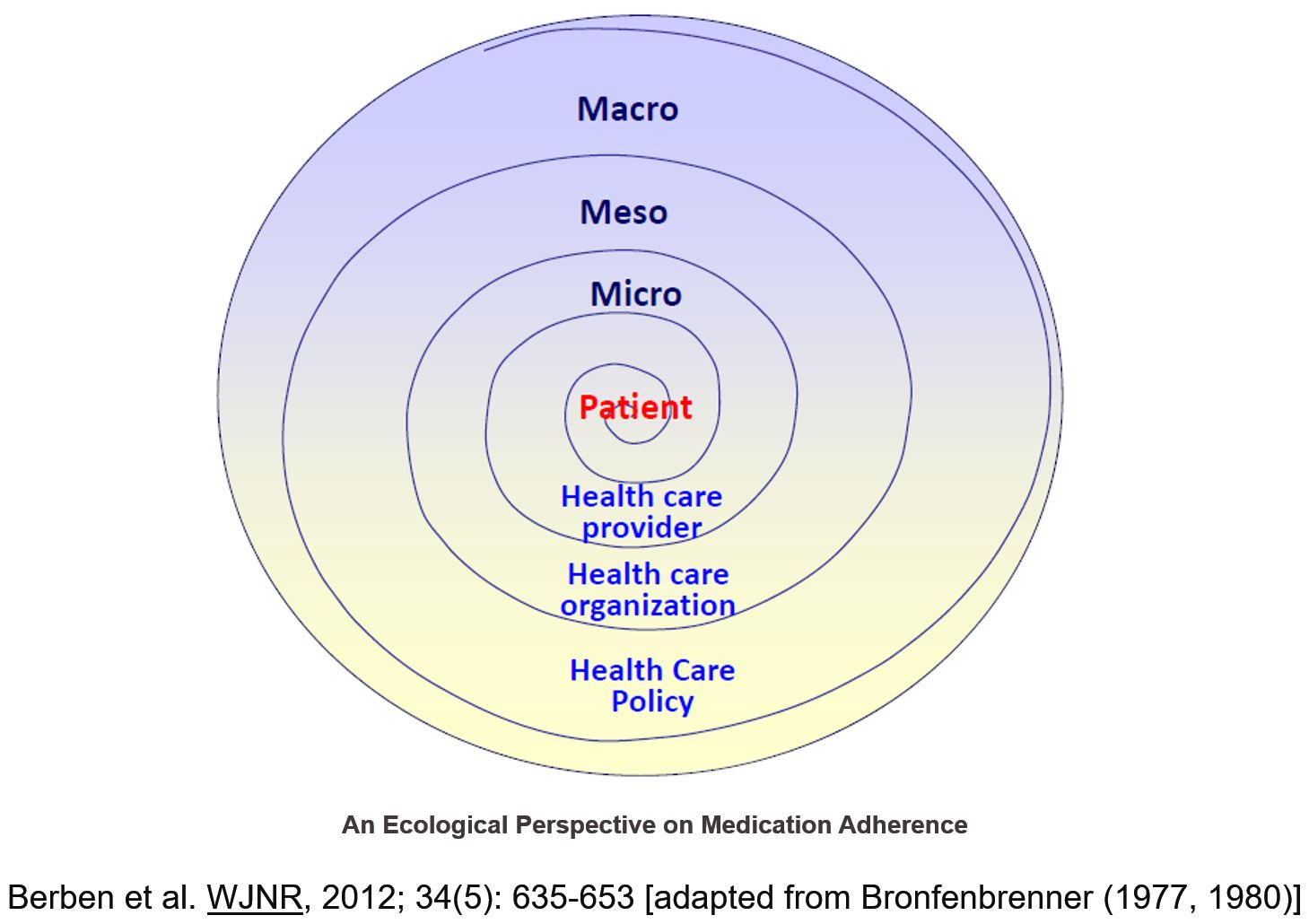
The unaltered long-term prognosis after heart transplantation underscores an urgent need to identify and improve factors impacting long-term survival. The healthcare system (e.g. level of chronic illness management implemented) and patient self-management are major drivers of outcome improvement.
Aim
The primary aims of the Building research initiative group: chronic illness management and adherence in transplantation (BRIGHT) study of heart transplant patients are:
- To describe the practice patterns relating to chronic illness management at the participating centers, countries, and continents in heart transplantation (HTx)
- To assess the prevalence and variability of non-adherence to the post-HTx treatment regimen
- To determine the multi-level factors related to immunosuppressive medication non- adherence
- To benchmark the participating centers, countries, and continents in relation to chronic illness management practice patterns and non-adherence to the treatment regimen
Methods
The study uses a cross-sectional survey design in 36 heart transplant centers covering 11 countries in 4 continents. It uses 4 questionnaires; 1 for the director of each heart transplant center, 1 for the clinicians at each center, and 2 for each heart transplant recipient (interview and self-administered), in addition to collecting clinical information on each heart transplant recipient and a collateral report by the clinicians on patients’ medication adherence.